Novel reaction microscope scheme targets biologically relevant molecules
11 Nov 2016 by Simon Davies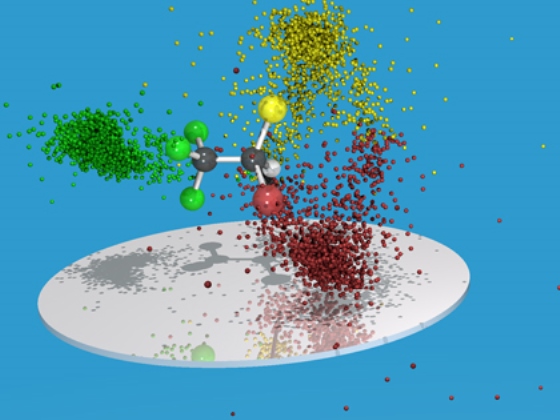
Latest spectrometer design is more sensitive to chemical fragments and can distinguish between isomers with more than one carbon atom
Researchers in Germany and the US have upgraded the performance of a reaction microscope so that the technique — known as Cold Target Recoil Ion Momentum Spectroscopy, or COLTRIMS for short — can be extended to distinguish between isomers with two carbon centres. Having demonstrated the applicability of the approach on a synthetic prototype molecule a few years ago, the advance allows the team to begin exploring more complex structures such as those found in healthcare, and further develop our fundamental understanding of their behaviour.
Molecules that are identical in composition, but occur as mirror images of each other can be challenging to identify directly using a single measurement. Typically, the determination of this so-called chirality (meaning handedness) requires additional information such as data from theoretical models or the use of reference substances where the handedness is already known. Pinpointing these subtle differences in structure is important as the two different versions (or enantiomers) of the molecule can behave differently – for example, in some cases only one of the enantiomers offers therapeutic properties. In other cases, one of the enantiomers could be toxic, while the other is harmless.
In their latest work, published in the Journal of Physics B: Atomic, Molecular and Optical Physics, the scientists have shown that the technique can be used to determine directly the configuration of halothane (CHBrClCF3) – a general anaesthetic.
To perform the measurement, the researchers bombard a gaseous sample with X-rays and look at the time taken for charged chemical fragments to reach the detector, as well as the position of the impacts. For instantaneously fragmenting molecules, the data provides the basis of a molecular fingerprint that can be related to a specific chemical structure: the left- and right-handed versions show up as two distinct peaks in the measurement output.
The group has worked hard to maximize the collection efficiency of its setup, and with good reason. As samples become more complex, the probability that fragmentation will produce neutral portions (which the detector is blind to) increases.
In this approach, at least four ions need to be detected to distinguish between the enantiomers and decipher the absolute configuration. In the case of halothane, the researchers collected the fragments CH+, Cl+, Br+, and CF+3 , coming from the same molecule.
“This result is an encouraging step towards application of the method to biologically relevant molecules,” commented team member Martin Pitzer, who is based at the University of Kassel, Germany.
Other institutions participating in the study include Johann Wolfgang Goethe-University Frankfurt, the University of Nevada and Philipps-Universität Marburg.