New handheld bioprinter holds promise for treating serious burns
04 Feb 2020 by Simon Davies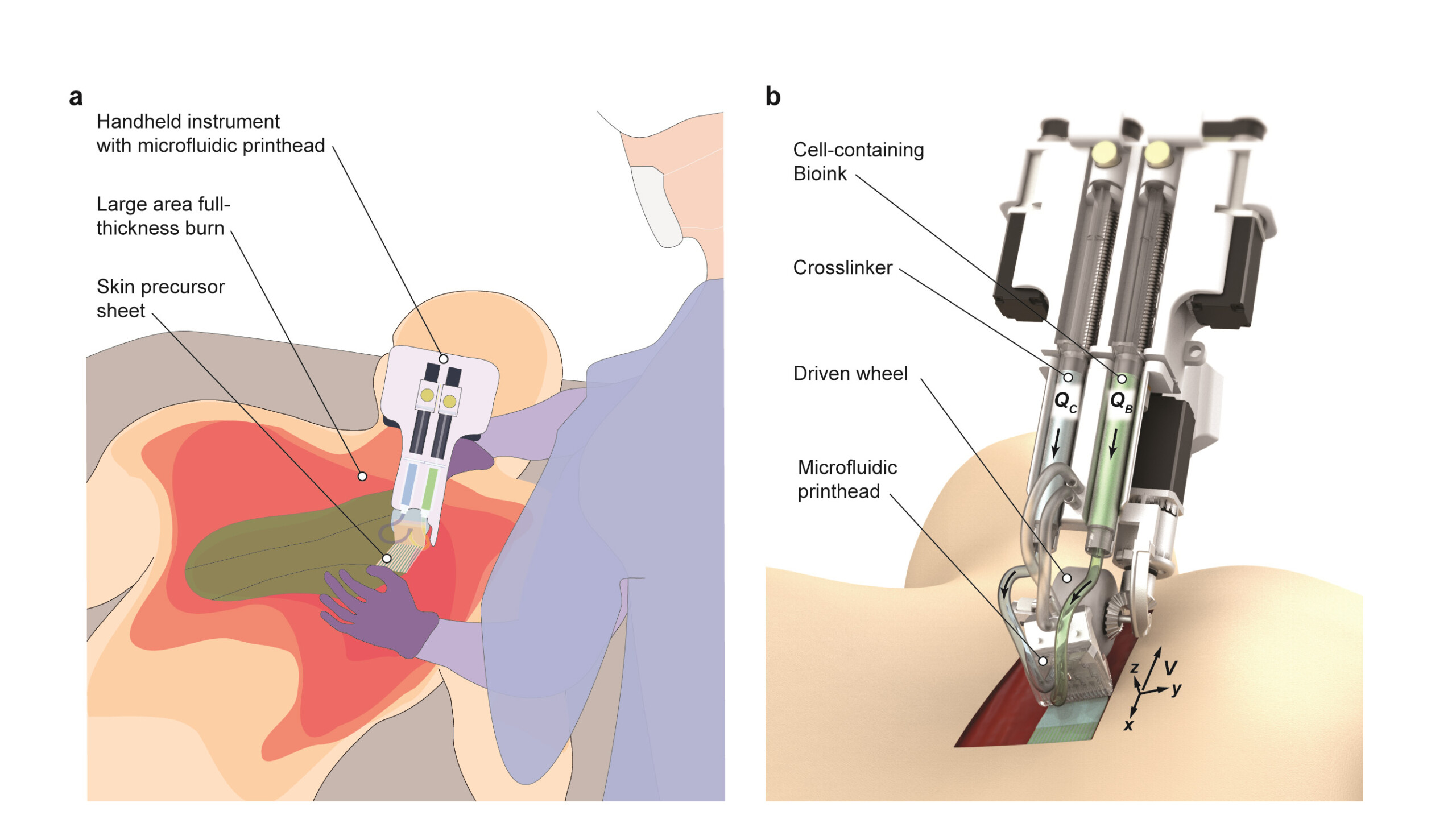 Left: a schematic illustration of how the team's device is used. Right: a rendered image of how the device delivers bioink directly onto the wound surface.
Left: a schematic illustration of how the team's device is used. Right: a rendered image of how the device delivers bioink directly onto the wound surface.
A team of researchers in Canada have successfully trialled a new handheld 3D skin printer, which treats severe burns by ‘printing’ new skins cells directly onto a wound.
Although the new system is in the early stages of development, it may eventually provide a way to treat patients whose burn injuries are too extensive to allow skin grafts. The results are reported today in the IOP Publishing journal Biofabrication.
Senior author Professor Axel Günther, from the University of Toronto, said: “Skin grafts, where the damaged tissue is removed and replaced with skin taken from another area of the patient’s body, are a standard treatment for serious burns.
“However, in cases where a patient has extensive full thickness burns – which destroy both the upper and lower layers of the skin – there is not always sufficient healthy skin left to use.
“While there are alternatives – including scaffolds using bovine collagen or engineered skin substitutes grown in vitro – none are ideal. Collagen scaffolds rely on tissues and cells surrounding the wound to fully heal, while in vitro skin substitutes can take many weeks to prepare, and are difficult to apply successfully to a patient when the burn area is large.”
To overcome these challenges, the research team designed a handheld device to deposit precursor sheets directly onto wounds of any size, shape or topography. It uses a bioink based on fibrin – a protein involved in the clotting of blood – infused with mesenchymal stromal cells (MSCs), which support the growth of local cells and assist in the body’s immune response. This is ‘printed’ directly onto the wound from the device’s soft roller.
Co-author Dr. Marc Jeschke, medical director of the Ross Tilley Burn Centre at Sunnybrook Health Sciences Centre in Toronto, said: “In general, the wound surfaces we designed this device for are not flat, nor are they oriented horizontally. One of the most important advantages of the device is that it should allow for the uniform deposition of a bioink layer onto inclined surfaces.
“In this study, we tested whether the device could do this effectively by using it to treat full-thickness burns in pigs. We found the device successfully deposited the ‘skin sheets’ onto the wounds uniformly, safely and reliably, and the sheets stayed in place with only very minimal movement.
“Most significantly, our results showed that the MSC-treated wounds healed extremely well, with a reduction in inflammation, scarring, and contraction compared with both the untreated wounds and those treated with a collagen scaffold.”
Professor Günther said: “We’re extremely pleased by this successful test. As well as the excellent healing outcomes, we’ve shown that the device offers a user-friendly method for safe cell and biomaterial delivery. Further study is required, but the signs are promising and the potential clinical applications for the device extend well beyond full thickness burn injuries.”